Water is the element that more than any other defines the identity of our planet. It covers over seventy percent of the Earth’s surface, regulates the climate, shapes geology, and forms the chemical basis of all known life. Yet, its origin is far from obvious. Understanding how primordial water arrived on Earth, why a similar planet like Mars almost completely lost it, and how water enabled the emergence of life means reconstructing a fundamental part of the history of the Solar System and planetary habitability.
When Earth formed around 4.5 billion years ago, the inner Solar System was an extremely hot and violent environment. For a long time, it was believed that under such conditions, water could not have been present from the start and must have been delivered later by bodies from colder regions. However, research over the past few decades has profoundly changed this view. Today we know that a significant portion of Earth’s water is of “internal” origin, meaning it was already present in the materials that formed the planet.
During Earth’s accretion, the planetesimals* (see below) that contributed to its growth contained hydrated minerals, in which water was chemically bound to the crystal structure. In the early stages, the planet experienced a global magma ocean phase. The intense heat caused degassing of the mantle, releasing large amounts of water vapor, carbon dioxide, and other gases. This created a dense primordial atmosphere, in which water vapor was a dominant component. As the surface gradually cooled, this vapor condensed and fell as persistent rains, forming the first oceans. The discovery of ancient zircon crystals, up to 4.4 billion years old, provides indirect but robust evidence of liquid water on Earth’s surface at a surprisingly early time.
To this internally derived water was added a crucial contribution from space. During the period known as the Late Heavy Bombardment, Earth was struck by numerous asteroids, particularly carbonaceous types rich in water and organic compounds. A key supporting element for this hypothesis is isotopic comparison: the ratio of deuterium to hydrogen in Earth’s water closely matches that measured in these asteroids. This indicates that a significant fraction of today’s ocean water comes from such impacts. Comets, although spectacular and ice-rich, appear to have played a secondary role. In most cases, their isotopic ratios do not match that of Earth’s water, suggesting their contribution was limited and not dominant.

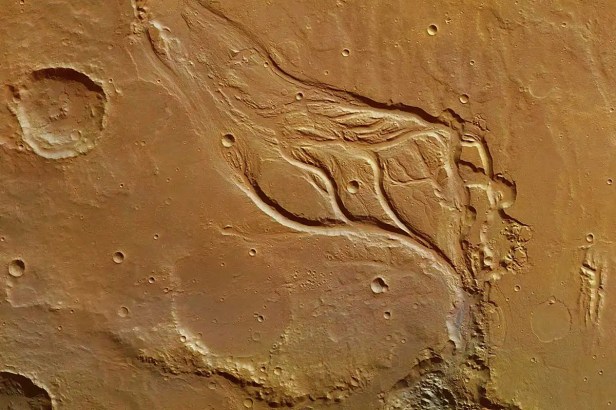
The story of water on Earth becomes even more instructive when compared to that of Mars. Images and data collected from space missions clearly show that the Red Planet once had rivers, lakes, and perhaps even oceans. Today, however, Mars is a cold and arid world. The main cause of this divergence lies in its smaller mass. Being much smaller than Earth, Mars lost internal heat more quickly, with decisive consequences for its evolution.
A key event in Mars’ history was the loss of its global magnetic field. Without this shield, the solar wind was able to strike the planet’s atmosphere directly, slowly but inexorably eroding it. Ultraviolet radiation dissociated water molecules in the upper atmosphere, and the lighter atoms were dispersed into space. As the atmosphere thinned, surface pressure dropped below the point at which liquid water can exist stably. Consequently, much of Mars’ water is now trapped as ice in the poles or underground, while a significant portion has been permanently lost. Mars thus demonstrates that initial water alone is not enough: mass, atmosphere, and a magnetic field are also required to retain it over time.
On Earth, by contrast, the persistence of liquid water created conditions for one of the most extraordinary events in natural history: the origin of life. Water is not merely a passive backdrop but a central actor in chemical processes. It is an exceptional solvent, capable of facilitating complex reactions, transporting molecules, and stabilizing biological structures such as proteins and nucleic acids. In early Earth environments, oceans, coastal pools, and hydrothermal vents functioned as natural chemical laboratories.
Special attention is given today to submarine hydrothermal vents, where hot, mineral-rich water emerges from the ocean floor, creating strong chemical and energy gradients. In these environments, the first metabolic reactions and pre-cellular structures may have developed. The “RNA world” hypothesis suggests that molecules capable of both storing information and catalyzing chemical reactions preceded DNA and proteins. Water provided the indispensable medium for these molecules to form, interact, and replicate. In a geologically short time, Earth already hosted microbial life, all descended from a common ancestor known as LUCA.
The history of water on Earth is thus a cosmic story, intertwining internal planetary processes, astronomical events, and fundamental chemical dynamics. It teaches us that habitability is not the result of a single factor but of a delicate and rare balance. By studying Earth, Mars, and other worlds in the Solar System, we learn not only how we arrived here but also where and how we might find, elsewhere in the universe, other planets capable of supporting life.
Planetesimals*
Planetesimals are the fundamental building blocks of planet formation. They are primitive solid bodies that formed in the disk of gas and dust surrounding the newborn Sun about 4.6 billion years ago and represent a crucial intermediate stage between cosmic dust and fully formed planets.
At the beginning of the Solar System, a vast cloud of gas and microscopic particles collapsed under gravity, giving rise to the Sun. Around it remained a protoplanetary disk, composed of mineral dust, ices, and gas. Within this disk, smaller particles began to clump together through collisions and electrostatic adhesion, forming progressively larger aggregates. When these aggregates reached kilometer-scale sizes, planetesimals were born.
Physically, a planetesimal is a body large enough to be dominated by its own gravity but too small to be considered a planet. Their sizes ranged from a few kilometers to hundreds of kilometers in diameter. They were extremely numerous and populated the entire early Solar System, moving in often unstable orbits and undergoing frequent collisions.
Planetesimals were not all the same. Their composition depended on their distance from the Sun. In the inner regions, where temperatures were high, planetesimals were mostly rocky and metallic. In the outer regions, beyond the so-called “snow line,” they could incorporate large amounts of water ice, ammonia, and methane. This compositional difference is crucial for understanding the origin of Earth’s water: many of the planetesimals that contributed to its growth contained water chemically bound in minerals or as ice.
Through a process called accretion, planetesimals collided and merged progressively, forming larger bodies known as protoplanets. Earth, Mars, and the other rocky planets are the final result of millions of years of planetesimal collisions. Some impacts were energetic enough to melt the forming planet completely, promoting mantle degassing and the release of primordial water.
Not all planetesimals were incorporated into planets. Many still survive today as asteroids, meteorites, and, in more distant regions, as comet nuclei. Meteorites falling on Earth are often fragments of extremely ancient planetesimals and provide one of the primary sources of direct information on the composition of the early Solar System.
In summary, planetesimals are the first large solid objects of the Solar System, the precursors of planets, and the main carriers through which fundamental materials – such as water and organic compounds – were distributed to forming worlds. Without planetesimals, Earth as we know it simply would not exist.
